If you’ve never heard of fibrodysplasia ossificans progressiva (FOP), you’re not alone. It’s one of those conditions that sits at the edge of medical awareness—so rare it can feel almost mythical—yet for the people who live with it, FOP shapes every decision, every plan, every movement. I’ve spent years speaking with families, clinicians, and researchers around FOP, and one theme always surfaces: the condition is as much about the weight of anticipation as it is about bone. Understanding how and why that happens can make this rare disease feel a little less impossible to grasp.
What FOP Is—and Why That Nickname Sticks
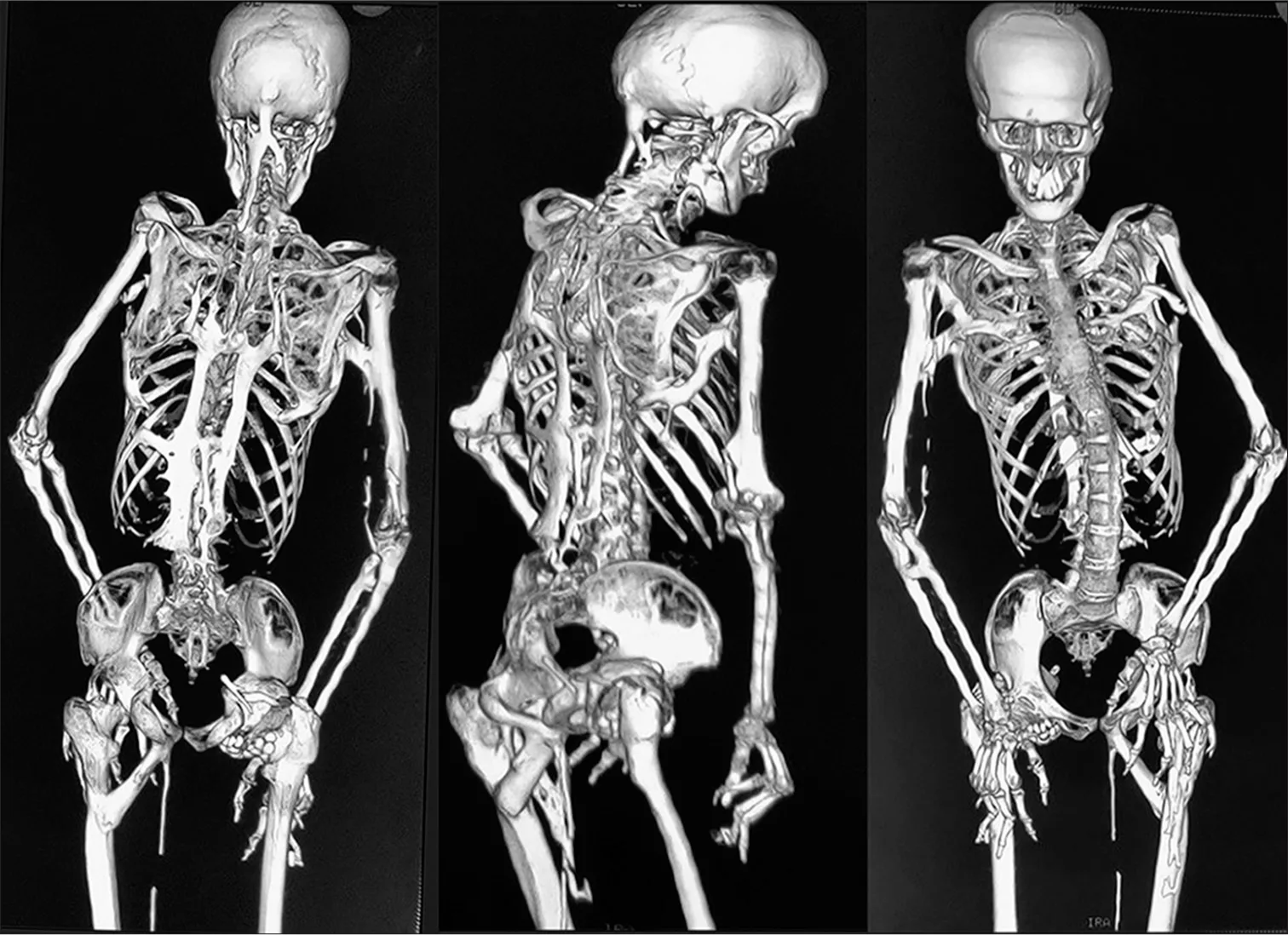
“Stone man syndrome” is the nickname that grabs headlines: startling, dramatic, and admittedly unhelpful. FOP is the accurate term. It describes a genetic disorder where soft tissues—muscles, tendons, ligaments, and fascia—gradually turn into mature bone through a process called heterotopic ossification (HO). Not calcification, not scar tissue—actual extra bone forming where it doesn’t belong.
A Few Basic Facts Paint the Picture:
- It’s ultra-rare. Estimates range from about 1 in 1 to 2 million people worldwide. Globally, there may be only several hundred to a thousand diagnosed individuals.
- It’s typically caused by a single DNA “spelling change” in a gene called ACVR1. That tiny change is enough to reroute normal repair signals toward making bone.
- It’s progressive and cumulative. Each flare-up of inflammation can leave behind new bone. Over years, this can lock joints and restrict movement.
- It spares some tissues. Smooth muscle (like the intestines), the heart, the tongue, and the diaphragm are not converted to bone. But chest wall muscles can ossify, which severely limits breathing mechanics.
The other reason that nickname sticks? Because the new bone is permanent. Once heterotopic bone forms, it doesn’t melt away.
A Rare Disease with a Familiar Arc: Discovery, Denial, and Adaptation
Ask families how FOP first showed up, and many will tell you the clues were there from day one. The big toe—short, curved, missing a joint, angled inward—is often the first sign. That malformation is so characteristic that when radiologists and pediatricians recognize it, they’re already most of the way toward a diagnosis.
Early Symptoms and Diagnostic Challenges
Early FOP can also masquerade. Swelling and hard lumps in a young child’s neck or back sometimes lead to panicked biopsies for suspected cancer. Those biopsies don’t help; they can trigger more bone formation and accelerate disability. I’ve heard parents describe a rollercoaster: urgent referrals, frightening possibilities, then a genetic test that explains everything and nothing all at once.
Navigating the Journey
It’s not a tidy journey. FOP moves in flares—periods of inflammation, pain, heat, swelling—followed by quiet stretches. Some people have frequent flares in childhood and relatively fewer in adulthood. Others experience long periods of stability interrupted by sudden, unexpectedly severe episodes. The uncertainty is a feature of the condition, not a bug.
How FOP Actually Turns Muscle Into Bone
You can’t pursue better treatments if you don’t understand the mechanism, and FOP researchers have spent decades chiseling away at this mystery.
The Underlying Mechanism
Here’s the short version of a very complex story:
- ACVR1 (also called ALK2) is a receptor on cell surfaces that normally responds to bone morphogenetic proteins (BMPs). Those signals help guide normal bone and cartilage development and repair.
- In FOP, a specific ACVR1 mutation—most commonly the R206H variant—acts like a stuck switch. It overreacts to signals and, strangely, begins to respond to Activin A (a growth factor that shouldn’t drive bone formation).
- When tissue is injured or inflamed, the immune system releases a soup of signaling molecules. In FOP, that environment flips skeletal progenitor cells into a chondrogenic and then osteogenic program—first cartilage, then bone.
- The new bone forms via endochondral ossification, the same process the body uses to lengthen bones in childhood.
Critical Insights
Two critical ideas come out of this:
- Injury matters. The more tissue trauma, the higher the risk of a flare and subsequent bone. This explains why invasive procedures can worsen the condition.
- The disease is genetically primed. Even minor inflammation can trigger outsized responses.
What FOP Looks Like Across a Lifetime
Every case is unique, but the condition follows certain patterns. Knowing these doesn’t predict any one person’s future; it does, however, give a sense of the road ahead.
Typical Progression
- Congenital sign: Most babies with FOP have malformations of the great toes at birth—shortened, inward-angled, and often with a missing joint.
- Early flares: Neck, back, and shoulders are common early sites. Lumps can be tender, warm, and sometimes bruise-colored. These may be mistaken for infection or tumors.
- Progression: As HO accumulates, joint mobility narrows. Knees and hips lose range. The jaw may stiffen, making dental care and eating difficult. The chest wall can become rigid, reducing lung expansion.
- Respiratory challenges: As thoracic movement becomes restricted, breathing depends more on the diaphragm. People may become prone to respiratory infections and fatigue because their chest can’t “pump” efficiently.
- Function: Mobility aids become a part of life; activities of daily living can require creative workarounds or assistance. Many people with FOP adapt impressively, continuing careers, relationships, and pursuits within the condition’s changing boundaries.
- Longevity: Published estimates vary, and individuals often outlive statistics. Respiratory complications and infections are leading causes of serious illness. Some live into their 50s or beyond; others face life-threatening complications earlier. The variability is striking.
Why FOP is Often Misdiagnosed—and How That Can Change Trajectories
FOP is rare, so many clinicians simply never encounter it in training. That’s the starting point for a cascade of errors:
- Soft-tissue masses in children raise alarms for cancer. Biopsies, though well-intentioned, can spark new HO.
- Intramuscular injections, deep tissue manipulations, or aggressive therapies may trigger flares.
- Early X-rays can be normal because heterotopic bone hasn’t matured yet.
Improving Diagnosis
The single most useful diagnostic clue is still the great toe malformation. When paired with a typical flare pattern and swelling in classic locations (neck, back, shoulders), suspicion should be high. A confirmatory genetic test for the ACVR1 mutation is widely available and doesn’t require invasive procedures.
Awareness campaigns led by patient organizations have helped change the narrative in pediatric and orthopedic circles. Radiology checklists now flag toe anomalies. Geneticists and rare disease clinics have pathways for rapid testing. The earlier FOP is recognized, the fewer the unnecessary procedures.
Language Matters: Why “Stone Man Syndrome” Falls Short
Words carry weight. “Stone man” dehumanizes and simplifies something profoundly complex. It suggests immobility and permanence, when in reality people with FOP often navigate life with creativity and humor. It also misses the variability: not everyone progresses at the same pace, and living with FOP isn’t a straight line toward immobility. Families often prefer FOP or the full name because it centers the medical truth without the tabloid edge.
The Same Mutation, Different Lives: What Drives Variation?
Most people with FOP share the same ACVR1 mutation, yet their experiences diverge. Why? Researchers point to:
- Modifiers in other genes that fine-tune immune signaling or inflammatory responses.
- Environmental exposures and life events that increase or reduce tissue injury.
- Differences in healthcare access, including early diagnosis and experienced care teams.
This is why international registries matter. The FOP community has rallied around data sharing—tracking flare frequency, triggers, lung function, and functional outcomes—so patterns can emerge and inform better studies.
The Lived Experience: What Daily Life Actually Feels Like
If you’re picturing constant pain and immobility, that’s not the full story. Yes, flares can be excruciating. Swelling and muscle spasms can derail sleep, work, and school. But there are also quiet seasons. I’ve heard people describe their bodies like tectonic plates: still for months, then a sudden shift.
Real-Life Snapshots
A few snapshots from conversations over the years:
- A teenager keeping careful mental maps of crowded hallways—not out of fear, but strategy—choosing routes that minimize jostling.
- An adult who times big projects for “stable months,” when flares are less likely, while mentally budgeting for downtime.
- Parents who describe the “invisible labor” of scheduling medical visits around growth spurts and flu season, each a potential flare accelerator.
FOP threads through every ordinary task—washing hair, getting into cars, sitting in theater seats. It’s not only the big events that present challenges; it’s a thousand small design choices in a world built for bodies that bend.
Complications Ripple Outward: Breathing, Nutrition, and Sleep
When we talk about FOP, the visible ossification dominates the conversation, but the systemic effects matter just as much.
- Thoracic insufficiency: As the rib cage loses flexibility, oxygenation can suffer, especially during exertion or infections. People describe an “always shallow” feeling—like every breath is taken against a heavy sweater.
- Airway management: Limited jaw opening and neck movement complicate anesthesia and intubation. Specialized teams often plan ahead, using techniques that reduce trauma risk.
- Nutrition: Jaw stiffness can make chewing difficult. Maintaining adequate calories and protein becomes a logistical puzzle, especially during growth or illness.
- Sleep: Pain and breathing effort can fragment sleep. Daytime fatigue follows. Some individuals develop sleep-disordered breathing or need ventilation support strategies tailored to their anatomy.
These aren’t side stories; they’re central to day-to-day function and long-term health.
The Drug Landscape: What’s Approved, What’s Experimental
For decades, management of FOP focused on avoiding trauma, quelling inflammation when flares hit, and supporting function. The research tide changed when scientists pinpointed the ACVR1 mutation and Activin A’s rogue role. That opened doors.
- Palovarotene (brand name Sohonos): A retinoic acid receptor gamma (RARγ) agonist, designed to interrupt the cartilage-to-bone pathway. In 2023, U.S. and Canadian regulators approved it to reduce new HO in certain age groups. Because retinoids can prematurely close growth plates and cause birth defects, the drug carries strict safety requirements. Access and coverage vary by country, and specialists monitor for side effects that include dryness of skin and mucosa, lipid changes, and liver enzyme elevations. Not all regions have authorized it; some regulatory agencies in Europe have not granted approval as of late 2024.
- Corticosteroids: In many clinical settings, short bursts are used at the start of severe flares affecting major joints. The evidence base is modest, and practice varies among centers. The approach aims to tamp down the initial inflammatory surge.
- Pain management: Multimodal regimens are common, blending medications that target neuropathic and inflammatory components. Simple-sounding issues—like managing constipation from opioids—become more complicated when mobility is limited.
- Experimental avenues: Researchers have probed several strategies:
- Blocking Activin A with monoclonal antibodies.
- Inhibiting ALK2 (ACVR1) signaling with small molecules.
- Repurposing kinase inhibitors with antifibrotic properties.
- Retinoid pathways, as with palovarotene and related compounds.
- Modulating the immune response at flare onset.
- Gene and RNA-based therapies that correct or silence the defective signal.
Some programs have shown promise but faced safety concerns; others didn’t meet endpoints. This is the grind of rare disease research: each study adds knowledge, even when it doesn’t deliver the hoped-for effect.
Surgery Is Not a Solution
Surgical removal of heterotopic bone almost always triggers rapid regrowth—often worse than before—unless the underlying biology is controlled. That’s why elective surgery is generally avoided in FOP. There are emergencies and unavoidable scenarios where procedures happen, but the bar is high, the planning is meticulous, and teams weigh the risks against the absolute necessity. This isn’t caution for caution’s sake; it’s born from decades of lived outcomes.
Kids Grow; FOP Changes the Rules
Childhood FOP is not just “adulthood FOP, smaller.” Growth plates, developing lungs, and rapidly changing biomechanics all alter disease dynamics. Some observations from pediatric-focused teams:
- Flares can cluster during growth spurts.
- Palovarotene’s growth-plate risks drive conservative use and strict age criteria in approvals; skeletal maturity matters.
- School participation often hinges on creative accommodations and awareness of the condition’s variability. Educators who understand that “good days” can flip without warning make a huge difference.
Families become experts in navigating a healthcare world that’s not designed for ultra-rare diseases. They also become brokers of knowledge—sharing experiences through foundations, registries, and private networks that often move faster than published literature.
The Money and Time Tax of Rarity
Rare diseases are expensive in ways that don’t show up neatly on bills:
- Travel to the handful of experienced centers adds costs for flights, hotels, time off work.
- Equipment needs change over time; what fits now may not work next year as range of motion shifts.
- Insurance coverage for off-label medications or specialized supports can be a marathon of appeals.
- Clinical trials, when available, may require frequent visits far from home.
Families describe entire calendars bent around medical logistics. Even when communities rally with fundraisers and practical help, the hidden costs—like caregiver burnout—accumulate.
Mental Health Isn’t Separate; It’s Intertwined
You can’t talk about FOP without acknowledging the mental and emotional load. Anticipating flares, managing pain, navigating inaccessible spaces, facing social isolation—these create a landscape where anxiety and depression can flourish. People also talk about unexpected sources of strength: peer communities that “get it,” clinicians who listen, creative outlets that restore a sense of agency, and the deep bonds that form in families who problem-solve together every day.
The Importance of Community Support
I’ve noticed a trend among adults with FOP who become advocates or mentors for younger patients. They’re realistic without being fatalistic. They speak clearly about loss and grief, and just as clearly about joy and purpose. That balance is its own kind of expertise.
The Ethics of Choice: Genetics, Family Planning, and Autonomy
FOP is autosomal dominant, which means an affected parent has a 50% chance of passing the mutation to each child. Most cases, though, occur as new mutations in families with no history. Genetic counseling gives space to explore options—natural conception, prenatal testing, preimplantation genetic testing with IVF, adoption—without prescribing a “right” pathway. People’s values differ, and the calculus is deeply personal.
Consent and Assent in Children
Another ethical dimension: consent and assent in children. Many interventions carry risks of triggering flares. Families and clinicians weigh potential benefits against the chance of harm, and as children grow, their voices should lead the discussion. That kind of shared decision-making respects both the science and the person living in the body.
Research is Sprinting and Slogging at the Same Time
The FOP field is a study in contrasts. On one hand, the path from gene discovery to a targeted approval happened within a few decades—a blink in biomedical time. On the other, making a meaningful dent in progression remains a formidable challenge.
Where Scientists Are Focusing
- Precision inhibitors for ACVR1 signaling that spare normal bone growth.
- Smarter biologics that block Activin A pathways without collateral damage.
- Early flare biomarkers—blood tests or imaging signals—to catch and modulate activity before HO is laid down.
- Delivery systems for gene editing or RNA-based approaches that reach the right skeletal progenitors without provoking the immune system.
- Better outcome measures: standardized ways to quantify HO volume, joint function, and quality of life that satisfy regulators and matter to patients.
Technical hurdles are nontrivial. The cells that give rise to HO are rare and scattered, the inflammatory microenvironment is noisy, and interventions must avoid normal skeletal maintenance. Yet each dataset from registries, each biobank sample, each carefully phenotyped patient helps refine the map.
Imaging: Seeing Bone Arrive Before It’s Bone
Traditional X-rays capture HO only after it mineralizes. Researchers now use:
- MRI to detect early edema, inflammation, and the cartilage “primordium” of future bone.
- Low-dose CT (and sometimes whole-body CT) to quantify HO burden and track changes over time.
- Nuclear medicine techniques, such as 18F-NaF PET, to detect active mineralization.
These tools don’t just document; they guide trials. If you can see the train leaving the station before it hits full speed, you can try to slow it down.
The Role of the Immune System
Inflammation is the match that lights the fuse, but it’s not just “more inflammation equals more bone.” The immune signature of FOP flares has a rhythm. Mast cells, macrophages, and lymphocytes cycle through early phases, releasing cytokines that push progenitor cells along the cartilage-to-bone track. Those same signals are essential for normal healing in everyone else. The goal is not to shut down immunity—it’s to redirect or temper it so the repair pathway doesn’t detour into ossification.
Nuanced Interventions
This nuanced view helps explain why some broad anti-inflammatory drugs underwhelm and why precisely tuned interventions might fare better.
The Invisible Architecture of Care
When you peel back the curtain on “good FOP care,” you typically find:
- A clinician who has seen enough cases to recognize patterns and exceptions.
- A dentist and anesthesia team who plan around limited jaw opening and neck mobility.
- A radiologist who understands when imaging will change management and when it won’t.
- A social worker or patient navigator adept at unlocking coverage and support.
- A school or employer willing to adapt policies for an unpredictable condition.
These aren’t glamorous elements, but they make the difference between perpetual crisis mode and sustainable living.
Common Pitfalls in the FOP Journey—and Why They Keep Happening
Patterns repeat because systems teach them. A few examples:
- Diagnostic detours: When